As hundreds of Southeast Texans test positive daily with the Omicron variant reportedly responsible for more than 99% of new cases, COVID-19 mitigation and treatment options continue to emerge and evolve.
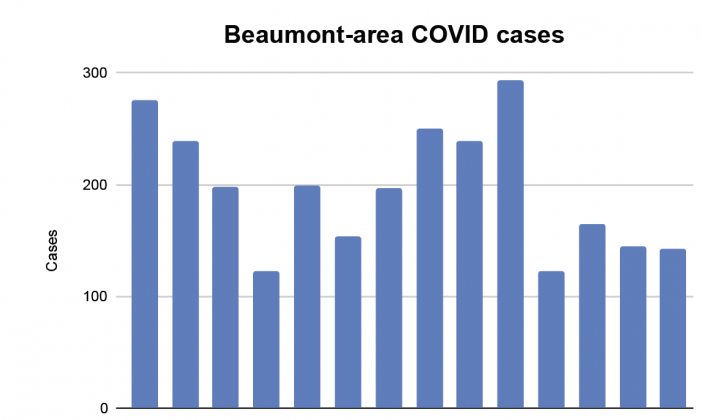
Even as 590 Beaumont-area residents tested positive from Jan. 23 to 26, the U.S. Food and Drug Administration (FDA) withdrew its use authorizations for two monoclonal antibody drugs locally used to treat COVID-19, Regeneron and Bam/Ete, which are reportedly not as effective in treating the Omicron variant.
According to a prediction presented by the Centers for Disease Control and Prevention (CDC), approximately 99.5% of the nation’s COVID cases last week were Omicron, while the other 0.5% of cases were attributed to Delta.
“This leaves Sotrovimab as the only monoclonal antibody therapy available to our Regional Infusion Center,” said Jefferson County Judge Jeff Branick. “Sotrovimab has been supplied to the State of Texas in very limited quantities and our anticipated weekly allocation in Southeast Texas will provide us with only enough antibodies to treat approximately 80 to 180 patients per week. The State of Texas has requested that the federal government allow it to purchase approved monoclonals directly from the manufacturers, but such request has not been acted upon by the feds as of yet.
“Because of these significant supply chain issues, the Regional Infusion Center will likely be able to remain open through this weekend at which time a decision will be made about hours and days of operation based upon then available medications.Effective immediately, much more strenuous screening will be initiated to ensure that the individuals most at risk of developing serious disease processes receive the infusion. We advise all individuals who test positive for COVID to visit a health care provider for information on COVID treatment options.”
Because data show these treatments are highly unlikely to be active against the Omicron variant, which is circulating at a very high frequency throughout the United States, FDA officials wrote, these treatments are not authorized for use in any U.S. states, territories, and jurisdictions at this time.
According to data from the Texas Department of State Health Services (DSHS), approximately 51% of eligible Jefferson County residents were fully vaccinated Jan. 26, while approximately 38% of Orange County residents and 40% of Hardin County residents could say the same. Approximately 74% of eligible Texas residents were fully vaccinated as of press time.
“While there are a few other monoclonals or pills which have proven effective, according to the FDA, none of these (Veklury, Paxlovid pill by Pfizer and molnupiravir pill by Merck) have been made available to our Regional Infusion Center or local pharmacies for administration,” said Branick. “We are advised that local pharmacies should start receiving the Pfizer and Merck pills within a week or so. A physician’s prescription will be required to get the Merck and Pfizer pills.”
While Pfizer’s COVID-19 treatment pills haven’t yet been made available to the Regional Infusion Center, it could be made available more broadly as an at-home treatment method to help reduce illness severity, according to a report by Pfizer officials.
“It has demonstrated potent antiviral in vitro activity against circulating variants of concern, as well as other known coronaviruses, suggesting its potential as a therapeutic for multiple types of coronavirus infections,” Pfizer officials wrote.
According to a report from the Southeast Texas Regional Advisory Council (SETRAC), area hospitals housed 354 COVID-positive patients, about 25% of its total admitted patients.